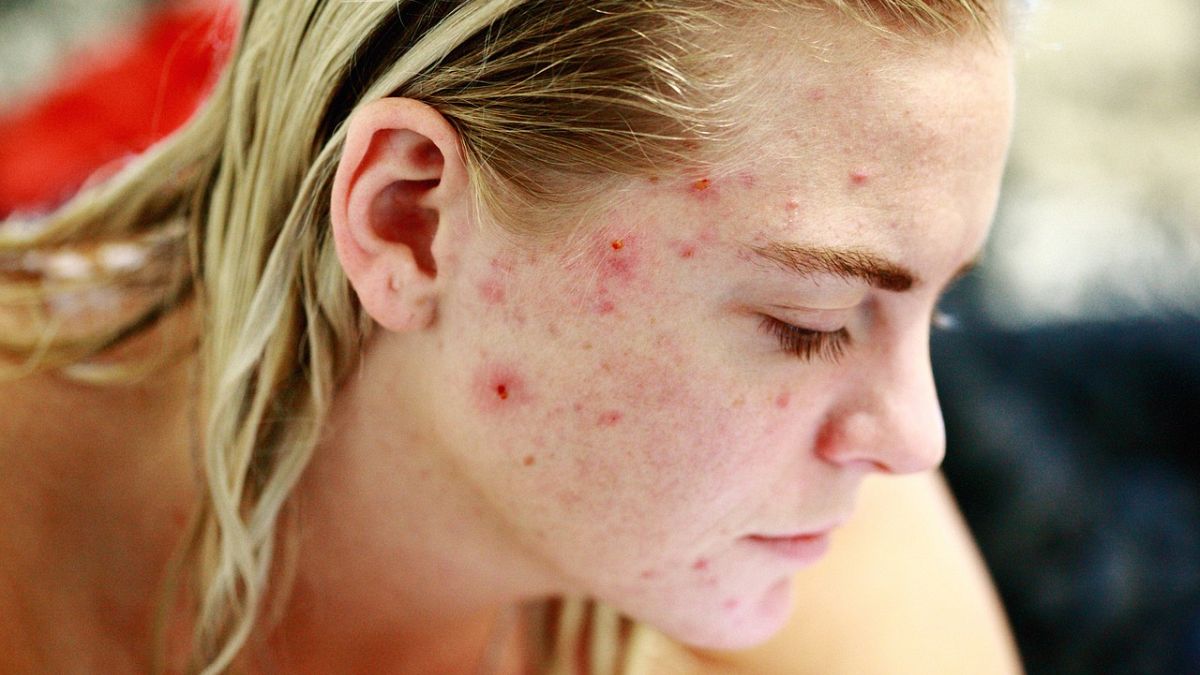
Scientists have developed a vaccine for the treatment of acne problems (acne), affecting about 95 percent of 11 to 30 people around the world.
If the vaccine, which is still in the testing phase, will pass through clinical trials, this will be the first vaccine against acne in the market.
Acne is a health problem that occurs with a clog of hair roots and pores on the skin and the reaction of the body to it, which usually leads to the formation of faces, upper hands and lesions on the back.
This happens, especially as a result of sensitivity to a change in hormones in puberty. Nevertheless, certain genetic factors, sensitivity to drugs or Acne Cutibacterium in the skin can also contribute to the problem of acne.
The pharmaceutical giant, based on French, began the early clinical tests of this vaccine for adults with an average -mimic acne deficiency.
The spokeswoman Sanofi Live Science said in his statement by e-mail, the new vaccine can “play a role in changing the treatment of acne,” he said.
Because patients with acne problems can be used for a long time and with serious side effects.
MRNA vaccine
The details of how the vaccine, which was developed in 2024, has not yet been divided.
Nevertheless, Sanofi says that this vaccine is MRNA vaccine.
MRNA vaccines work, giving genetic instructions (MRNA) of the pathogen using the disease. Thanks to these instructions, the cells learn to create a harmless part of the pathogen. The immune system recognizes this protein as a “foreign factor” and develops protection from it. Thus, when a real pathogen is found, the body is ready.
In this vaccine, target proteins will probably be proteins produced by C. Acnes. Sanofi said that the vaccine seeks to increase the patient’s immune response against certain types of bacteria, which is believed to contribute to the development of acne. C. Acnes is known as primary bacteria associated with acne.
Sanofi is currently known as FAZ I/II. It is expected that these experiments, which began in April 2024, will last until 2027. During this time, the company will add about 400 adults aged 18 to 45 years, who have problems with the middle to the middle of -4 on their faces.









Leave a Reply